PUBLICATIONS
2025
Expanding heterocyclic chemical space through the reactivity of N-allenamides
Dorian Schutz and Laurence Miesch
Comptes Rendus. Chimie, 2025, 28, 789-804 – DOI : 10.5802/crchim.414
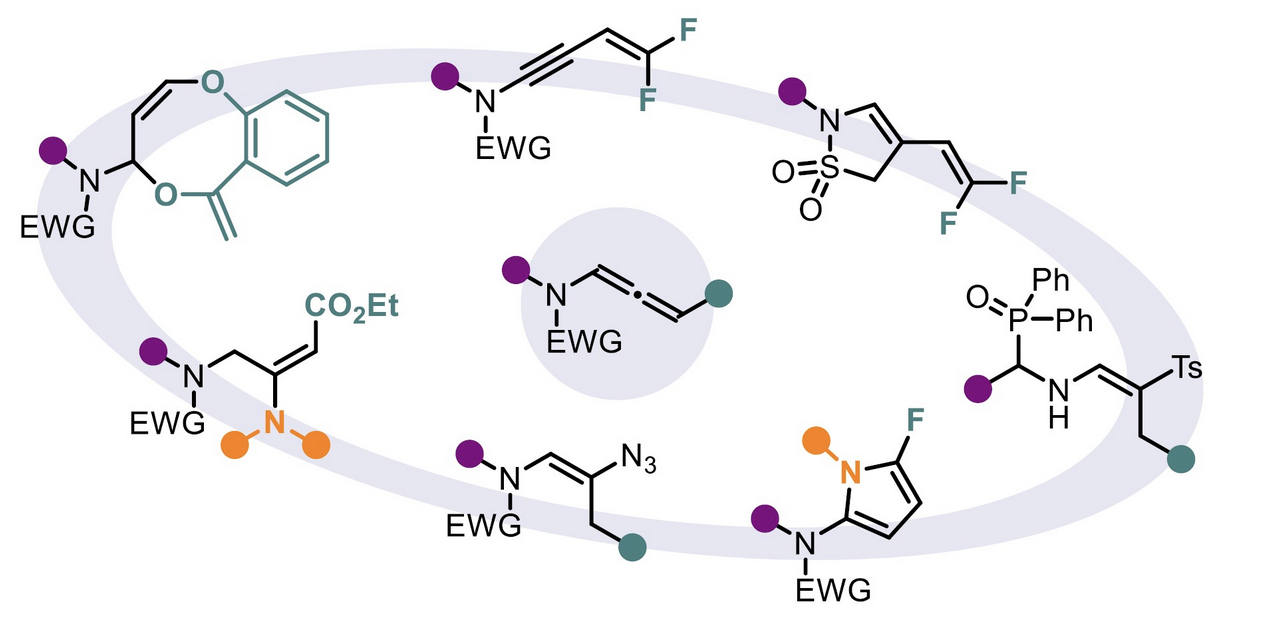
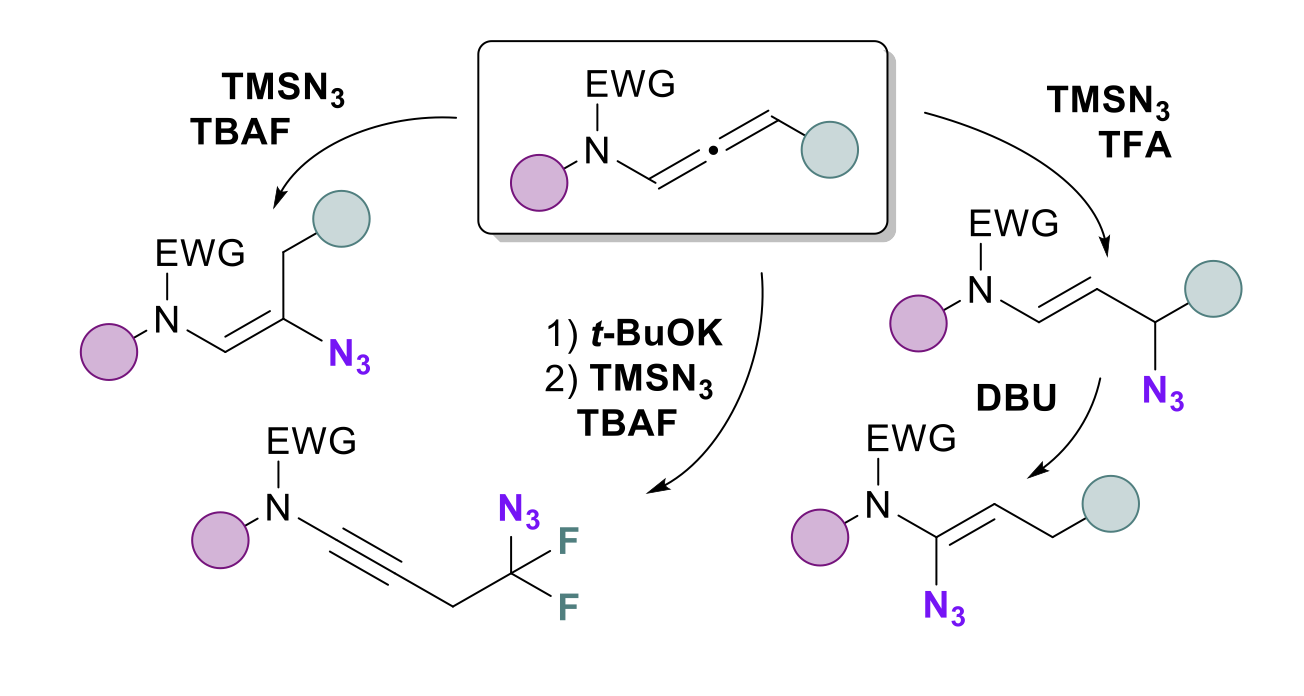
Regio-and Stereoselective Azidation of Activated N-allenamides: an entry to α, β, γ and δ-amido-azides
Dorian Schutz, Maxime Hourtoule and Laurence Miesch
Org. Chem. Front., 2025, 12, 185 – 191 – DOI: https://doi.org/10.1039/D4QO01802H
2024
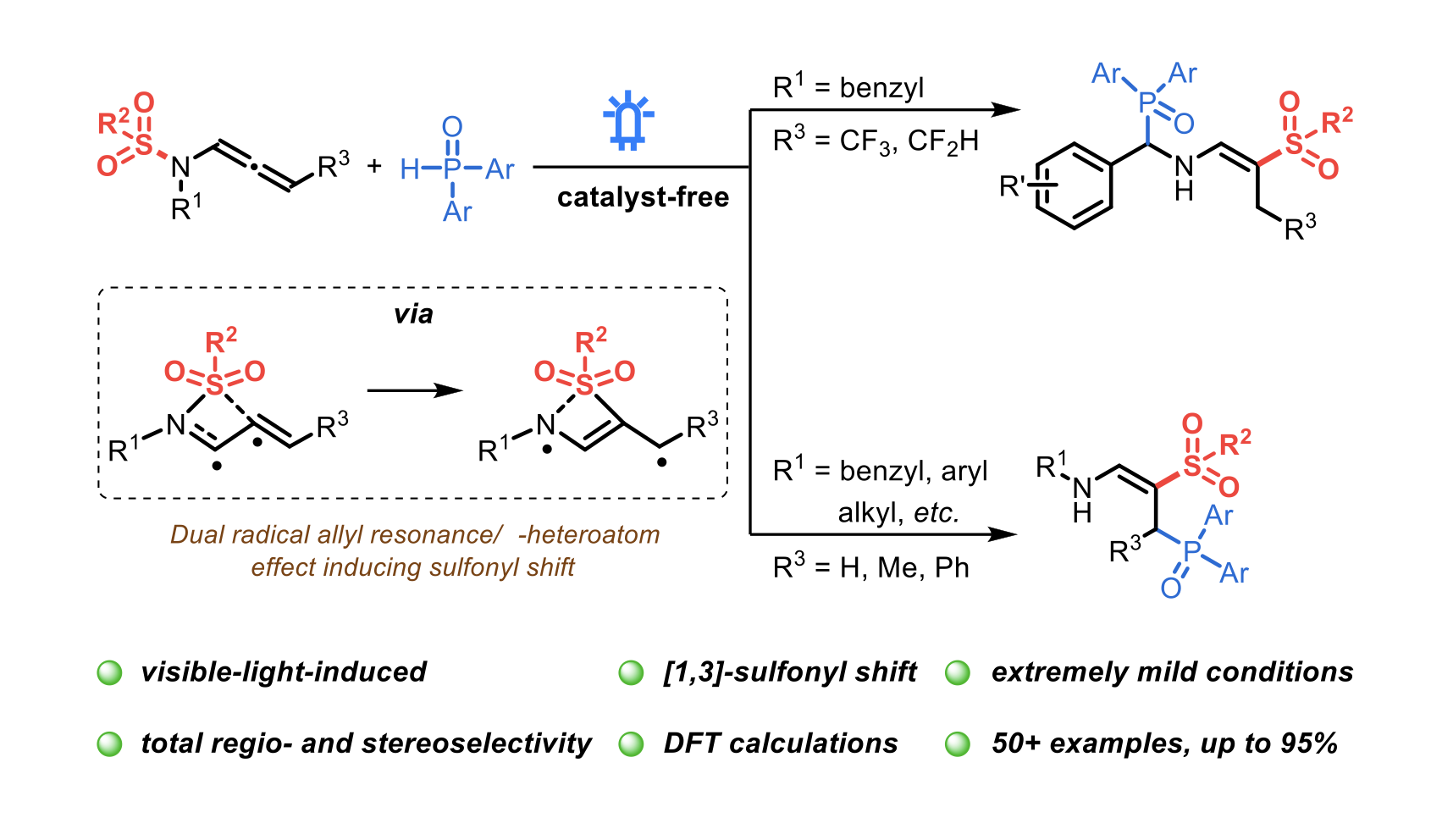
Photocatalyst-free, visible-light-induced regio- and stereoselective synthesis of phosphorylated enamines from N-allenamides via [1,3]-sulfonyl shift at room temperature
Jia-Dong Guo, Feven Alemu Korsaye, Dorian Schutz, Ilaria Ciofini and Laurence Miesch
Chem. Sci., 2024, (first published on 03 Oct 2024) – DOI: https://doi.org/10.1039/D4SC05190D
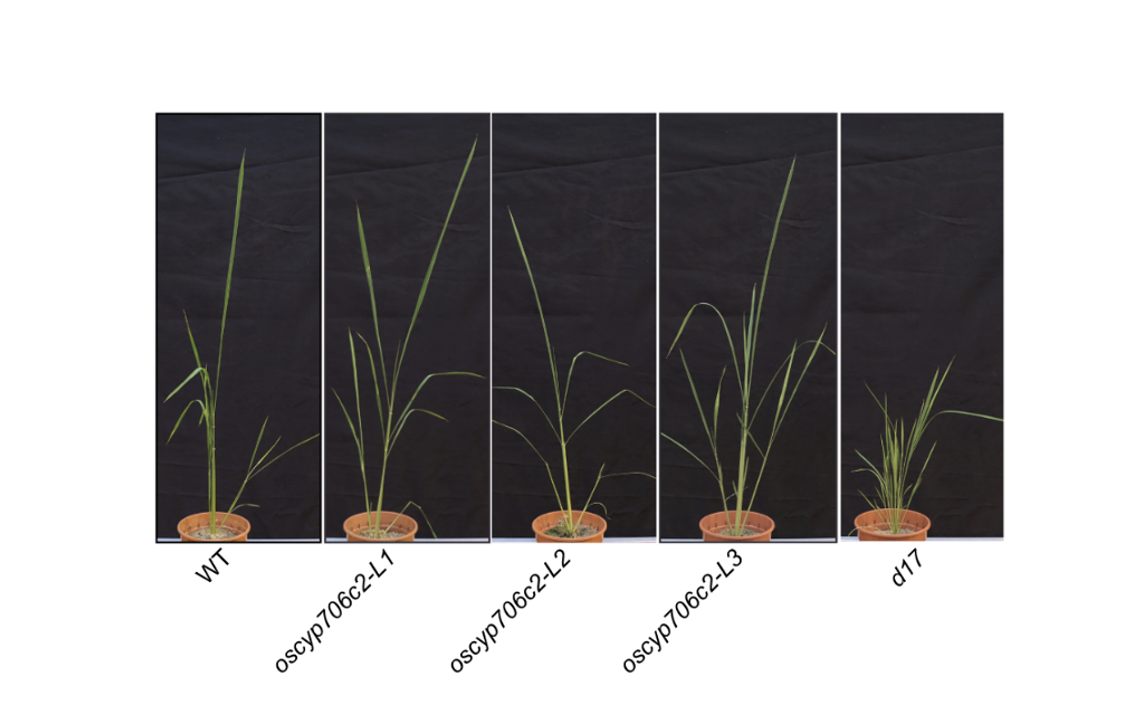
OsCYP706C2 diverts rice strigolactone biosynthesis to a noncanonical pathway branch
Changsheng Li, Imran Haider, Jian You Wang, Pierre Quinodoz, Hernando G. Suarez Duran, Lucía Reyes, Méndez Robin Horber, Valentina Fiorilli, Cristina Votta, Luisa Lanfranco, Samara M. Correia de Lemos, Lucile Jouffroy, Baptiste Moegle, Laurence Miesch, Alain De Mesmaeker, Marnix H. Medema, Salim Al-Babili, Lemeng Dong, and Harro J. Bouwmeester
Science Advances, 28 Aug 2024, Vol 10, Issue 35 – DOI: 10.1126/sciadv.adq3942
Transition Metal-Free Domino Hydroamination/Isomerization/Transamidation Sequence: An Entry to Trifluorinated γ-Lactams
Dorian Schutz, Clément Gommenginger, Baptiste Moegle, Maxime Hourtoule, Ludovik Noël-Duchesneau, and Laurence Miesch
J. Org. Chem. 2024 – DOI : https://pubs.acs.org/doi/10.1021/acs.joc.4c00878
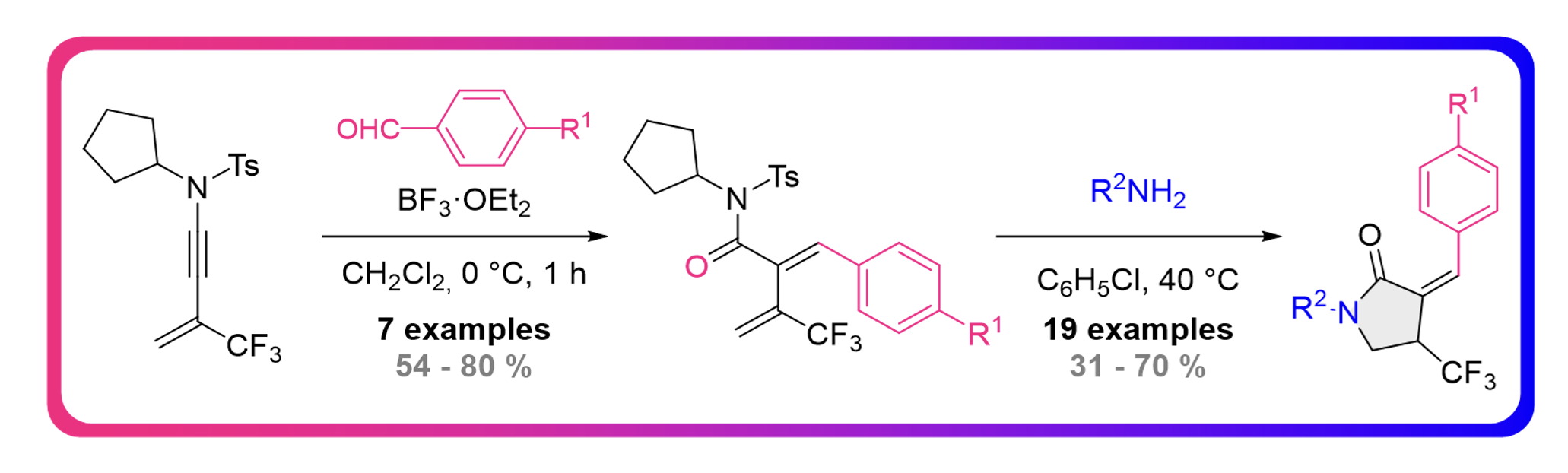
Metal Free Regio – and Stereoselective Semireduction of CF3-Substituted N-Allenamides
Clément Gommenginger, Maxime Hourtoule, Marco Menghini and Laurence Miesch
Org. Biomol. Chem., 2024, Advance Article – DOI : https://doi.org/10.1039/D3OB01859H
2023
Evolutionary metabolomics of specialized metabolism diversification in the genus Nicotiana highlights N-acylnornicotine innovations
David Elser, David Pflieger, Claire Vilette, Baptiste Moegle, Laurence Miesch, and Emmanuel Gaquerel
Science Advances, Vol 9, Issue 34, 2023 – DOI: 10.1126/sciadv.ade898
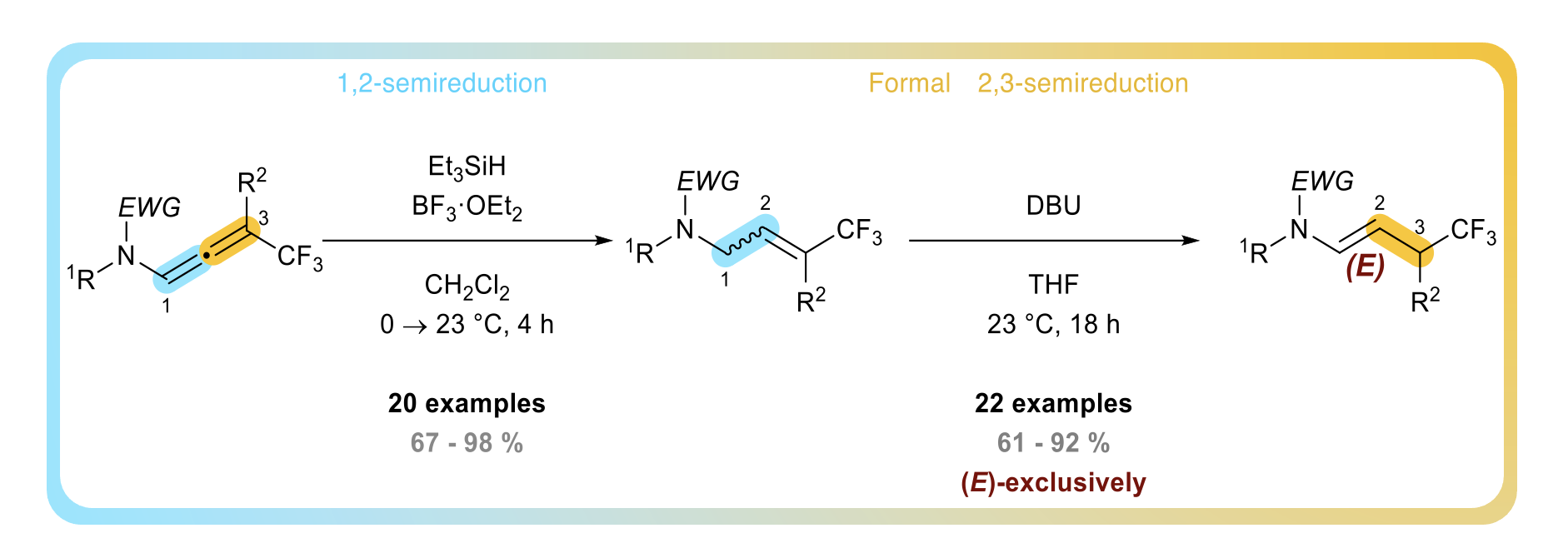
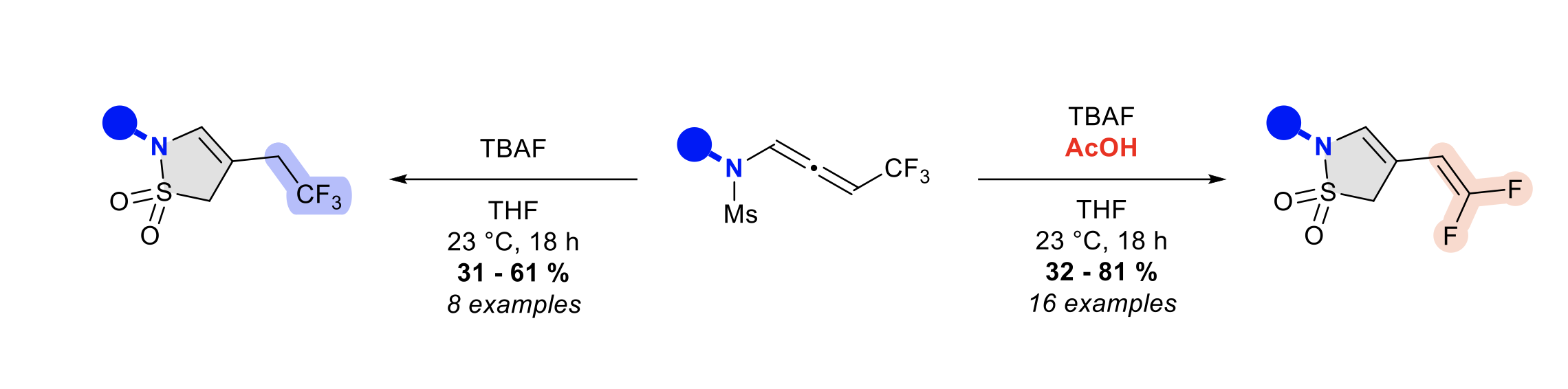
TBAF-promoted carbanion mediated sulfonamide cyclization of CF3-substituted N-allenamides an access to fluorinated gamma-sultams
Clément Gommenginger, Yongxiang Zheng, Daniele Maccarone, Ilaria Ciofini and Laurence Miesch
Organic Chemistry Frontiers , 2023 (invited paper) – DOI: 10.1039/D3QO00781B
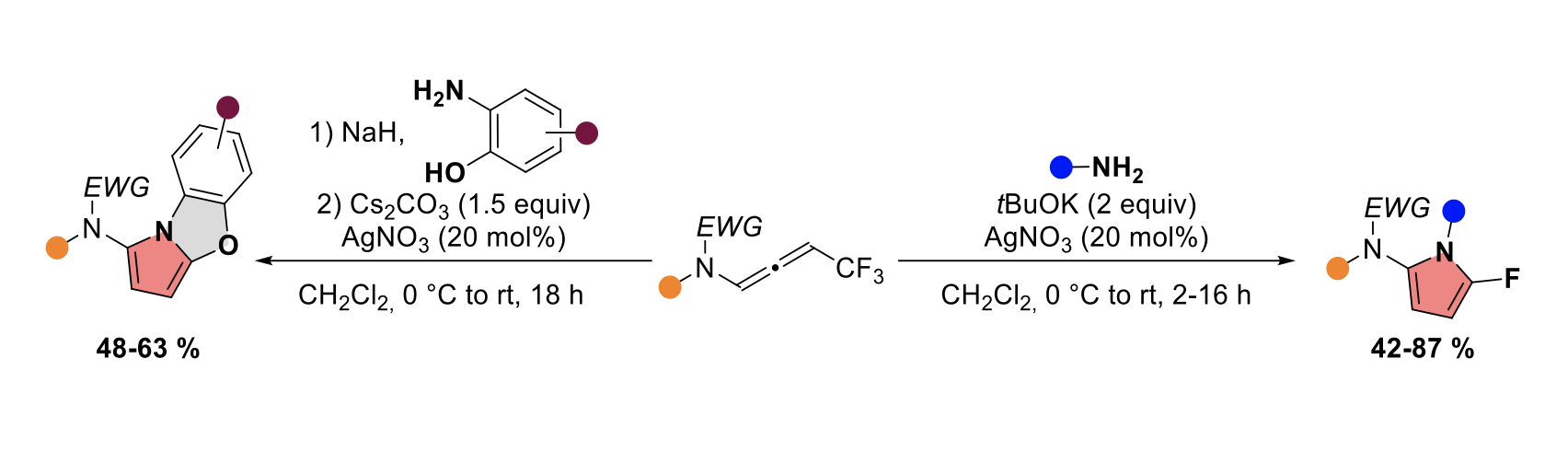
Silver-Catalyzed Domino Reaction of CF3Substituted N-Allenamides with Primary Amines for the Construction of 2-Amido-5-fluoropyrroles
Maxime Hourtoule and Laurence Miesch
Org. Lett., 2023 – DOI : https://doi.org/10.1021/acs.orglett.3c00401
2022
Construction of C-N and C-O bonds based on N-allenamide functionalization
Maxime Hourtoule and Laurence Miesch
Org. Biomol. Chem., 2022 -DOI : https://doi.org/10.1039/D2OB01768G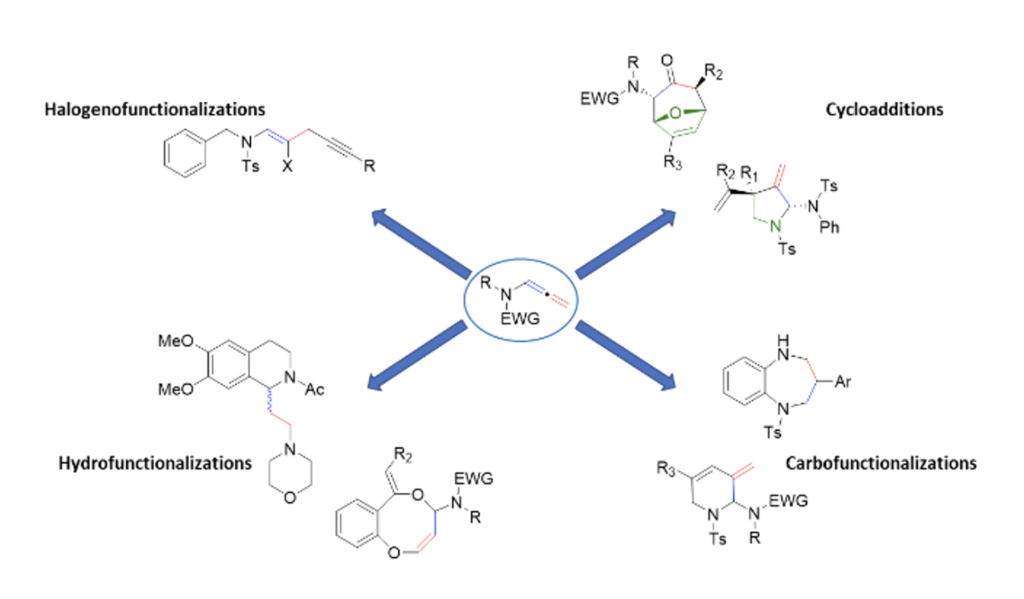
Noncanonical Strigolactone Analogues Highlight Selectivity for Stimulating Germination in Two Phelipanche ramosa Populations
Suzanne Daignan Fornier, Alexandre de Saint Germain, Pascal Retailleau, Jean-Paul Pillot, Quentin Taulera, Lucile Andna, Laurence Miesch, Soizic Rochange, Jean-Bernard Pouvreau, and François-Didier Boyer
Nat. Prod. 2022 – DOI: https://doi.org/10.1021/acs.jnatprod.2c00282
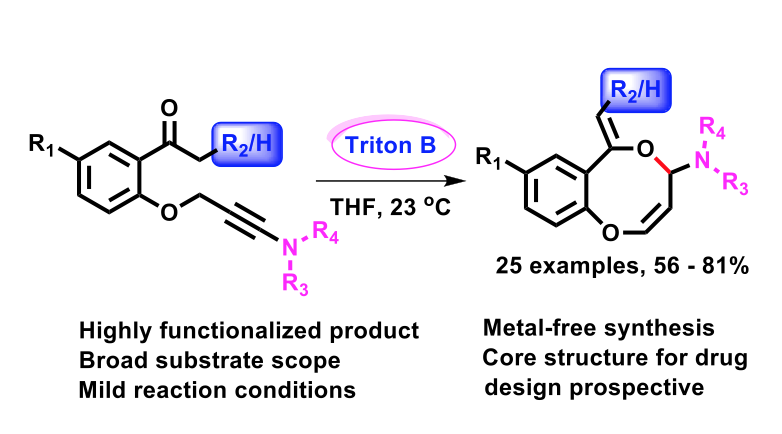
Triton B-Promoted Regioselective Intramolecular Addition of Enolates to Tethered Ynamides for the Construction of 8-Membered O-Heterocycles
Santanu Ghosh, Yongxiang Zheng, Daniele Maccarone, Feven Alemu Korsaye, Ilaria Ciofini, and Laurence Miesch
Org. Chem. Front., 2022 – DOI : 10.1039/D2QO00873D
Regio- and Stereoselective Addition to gem-Difluorinated Ene–Ynamides: Access to Stereodefined Fluorinated Dienes
Maxime Hourtoule and Laurence Miesch
Organic Letters, Articles ASAP (Letter), Publication Date (Web):May 19, 2022 – DOI: 10.1021/acs.orglett.2c01593
Synthesis of Non-natural Aza-Iridoids via Ynamides and Molecular Networking-Based Tracing of Their In Planta Bioconversion
Baptiste Moegle, Maxime Hourtoule, Clément Gommenginger, Asmaa Belabbes, Claire Villette, David Elser, Emmanuel Gaquerel, Nicolas Navrot, and Laurence Miesch
J. Org. Chem. First published: 12 May 2022 – DOI: https://doi.org/10.1021/acs.joc.2c00445
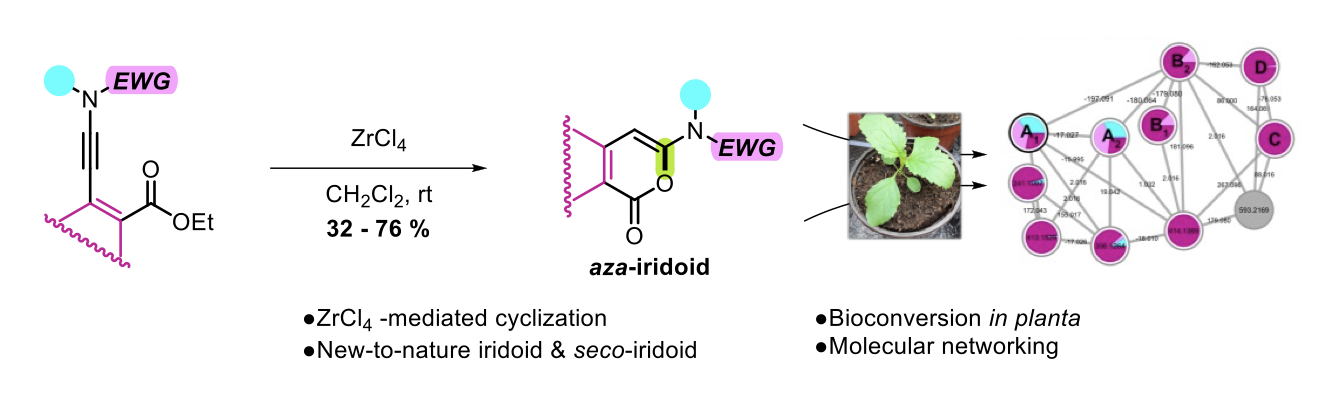
One-Pot anti-Michael Regio- and Stereoselective Hydroamination of Activated N-Allenamides
Maxime Hourtoule, Yongxiang Zheng, Anna Perfetto, Davide Luise, Ilaria Ciofini and Laurence Miesch
The Journal of Organic Chemistry, Publication Date (Web): March 28, 2022 – DOI : https://doi.org/10.1021/acs.joc.2c00302
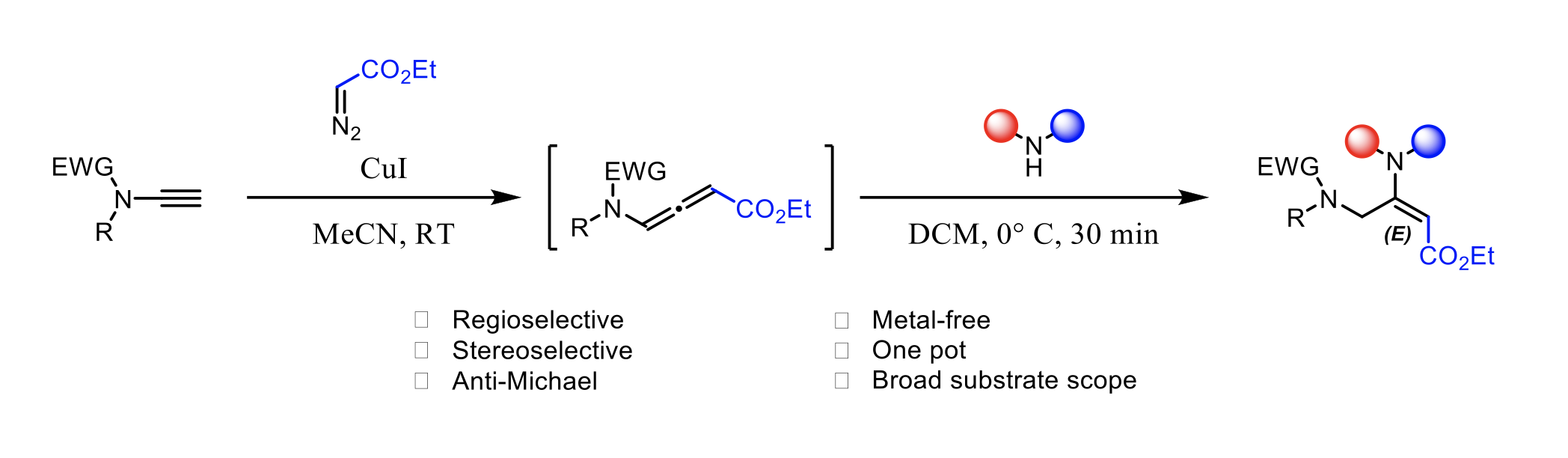
Copper-Catalyzed Synthesis of Terminal vs. Fluorine-Substituted N- Allenamides via Addition of Diazo Compounds to Terminal Ynamides
Yongxiang Zheng, Baptiste Moegle, Santanu Ghosh, Anna Perfetto, Davide Luise, Ilaria Ciofini and Laurence Miesch
Chemistry A European Journal, 2022 – DOI : https://doi.org/10.1002/chem.202103598
2021
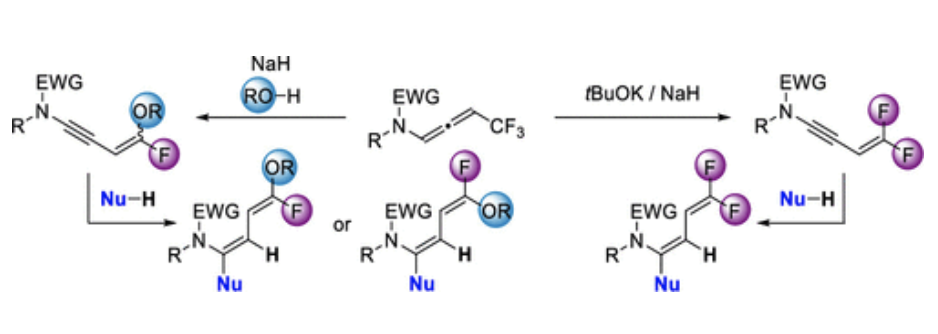
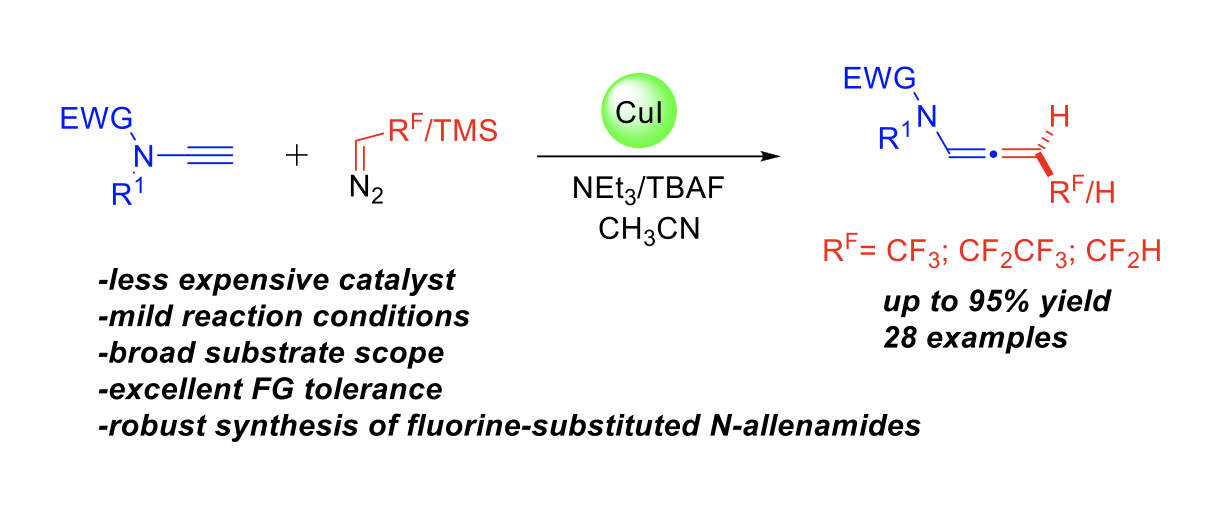
Direct Synthesis of CF2HSubstituted 2Amidofurans via Copper-Catalyzed Addition of Difluorinated Diazoacetone to Ynamides
Yongxiang Zheng, Anna Perfetto, Davide Luise, Ilaria Ciofini, Laurence Miesch
Organic Letters, 2021 – DOI : https://doi.org/10.1021/acs.orglett.1c01876
Innate promiscuity of the CYP706 family of P450 enzymes provides a suitable context for the evolution of dinitroaniline resistance in weed
Fatemeh Abdollahi, Mohammad Taghi Alebrahim, Chheng Ngov, Etienne Lallemand , Younxiang Zheng, Claire Villette, Julie Zumsteg, François André, Nicolas Navrot, Danièle, Werck‐Reichhart, Laurence Miesch
New Phytologist 2021, 229, 3253-3268 – DOI : 10.1111/nph.17126
2020
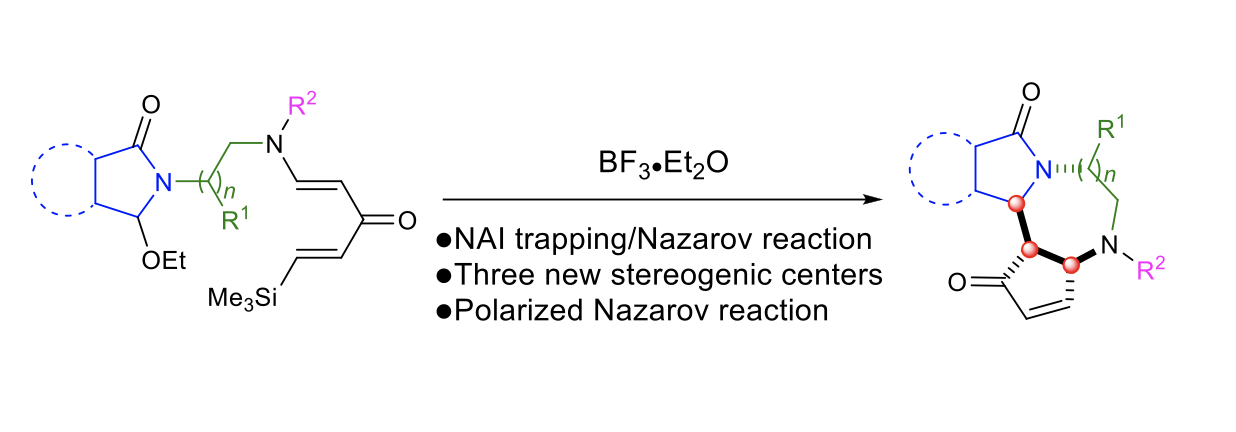
Tertiary Enamide-Promoted Diastereoselective Domino: N-Acyliminium Ion Trapping and Nazarov Cyclization
Yongxiang Zheng, Lucile Andna, Olivia Bistri, and Laurence Miesch
Org. Lett. 2020 – DOI : 10.1021/acs.orglett.0c02251
Tertiary Enamides as Versatile and Valuable Substrates to Reach Chemical Diversity
(invited review by professor Paul Knochel)
F. Beltran, L. Miesch
Synthesis 2020 – DOI: 10.1055/s-0040-1707403
Inhibition of phytosterol biosynthesis by azasterols
S. Darnet, L. Martin, P. Mercier, F. Bracher, P. Geoffroy, H. Schaller
Molecules 2020, 25, 1111 – DOI :10.3390/molecules25051111
2019
Surfactant Micelles Enable Metal-Free Spirocyclization of Keto-Ynamides and Access to Aza-Spiro Scaffolds in Aqueous Media
F. Beltran, A. V. Vela-Gonzalez, T. Knaub, M. Schmutz, M. P. Krafft, L. Miesch
Eur. J. Org. Chem. 2019 – DOI: 10.1002/ejoc.201901441
A Promiscuous CYP706A3 Reduces Terpene Volatile Emission from Arabidopsis Flowers, with Impacts on Florivores and Floral Microbiome
B. Boachon, Y. Burdloff, J.X. Ruan, R. Rojo, R. R. Junker, B. Vincent, F. Nicolè, F. Bringel, A. Lesot, L. Henry, J.E. Bassard, S. Mathieu, L. Allouche, I. Kaplan, N. Dudareva, S. Vuilleumier, L. Miesch, F. André, N. Navrot, X.-Y. Chen and D. Werck-Reichhart
Plant Cell, 2019 – DOI: 10.1105/tpc.19.00320
Characterization of Jasmonoyl-Isoleucine (JA-Ile) hormonal catabolic pathways in rice upon wounding and salt stress
M. Hazman, M. Sühnel, S. Schäfer, J. Zumsteg, A. Lesot, F. Beltran, V. Marquis, L. Herrgott, L. Miesch, M. Riemann and T. Heitz
Rice 2019 – DOI: 0.1186s12284-019-0303-0
Metal-Free Synthesis of Activated Ynesulfonamides and Teritary Enesulfonamides
L. Andna and L. Miesch
Org. Biomol. Chem. 2019, 17, 5688 – 5692 – DOI: 10.1039/C9OB00947G
Tertiary Enamide-Triggered SEAr: Domino Allylation and Enamine-Type Addition
F. Beltran and L. Miesch
Org. Lett. 2019, 21, 1569-1573 – DOI: https://doi.org/10.1021/acs.orglett.8b03987
Spirocyclization of Keto-Ynesulfonamides Promoted by Quaternary Ammonium Salts
Frédéric Beltran, Lucile Andna and Laurence Miesch
Org. Chem. Front. 2019, 6, 373-376 – DOI: 10.1039/C8QO00937F
Diastereo-/Enantioselective Diels-Alder Synthesis of 14b-Hydroxysteroid Scaffolds: A Combined Experimental and DFT Study
Clovis Peter, Philippe Geoffroy, Takatsugu Murata, Takayuki Tonoi, Isamu Shiina and Michel Miesch
Heterocycles, 2019 – DOI: 10.3987/COM-18-S(F)99
2018
Vitamin D5 in Arabidopsis Thaliana
Silvestro D, Villette C, Delecolle J, Olsen CE, Motawia MS, Geoffroy P, Miesch M, Jensen PE, Heintz D, Schaller H.
Sci Rep 2018, 8, 16348 – DOI: 10.1038/s41598-018-34775-z
Trapping of N-Acyliminium Ions with Enamides: An Approach to Medium-Sized Diaza-Heterocycles
Lucile Andna and Laurence Miesch
Org. Lett. 2018, 20, 3430-3433 – DOI: 10.1021/acs.orglett.8b01407
Intramolecular Morita-Baylis-Hillman reaction as a strategy for the construction of tricyclic sesquiterpene cores
Clovis Peter, Philippe Geoffroy and Michel Miesch
Org. Biomol. Chem. 2018, 16, 1381-1389 – DOI: 10.1039/C7OB03124F
Evidence for effective structure-based neuromodulatory effects of new analogs of neurosteroid allopregnanolone
Taleb, O., Patte-Mensah, C., Meyer, L., Kemmel, V., Geoffroy, P., Miesch, M., Mensah-Nyagan, A-G.
J. Neuroendocrinol. 2018 – DOI: 10.1111/jne.12568
Highly diastereoselective access to polyfunctionalized 1,3-oxazines promoted by Brønsted/Lewis acids
Clovis Peter, Philippe Geoffroy and Michel Miesch
Organic Chemistry Frontiers 2018, 5, 566 – DOI: 10.1039/C7QO00891K
2017
Social Isolation in Early versus Late Adolescent Mice Is Associated with Persistent Behavioral Deficits That Can Be Improved by Neurosteroid-Based Treatment
A. Locci, P. Geoffroy, M. Miesch; A-G. Mensah, G. Pinna
Frontiers in Cellular Neuroscience 2017, 11, article 208 – DOI: 10.3389/fncel.2017.00208
Direct Spirocyclization from Keto-sulfonamides: An Approach to Azaspiro Compounds
Frédéric Beltran, Indira Fabre, Ilaria Ciofini, and Laurence Miesch
Org. Lett. 2017, 19, 5042-5045 – DOI: 10.1021/acs.orglett.7b02216
Jasmonic Acid Oxidase 2 (JAO2) hydroxylates jasmonic acid and represses basal defense and resistance responses against Botrytis cinerea infection
Smirnova E, Marquis V, Poirier L, Aubert Y, Zumsteg J, Ménard R, Miesch L, Heitz T.
Mol Plant. 2017 – DOI: 10.1016/j.molp.2017.07.010
Allopregnanolone and its analog BR 297 rescue neuronal cells from oxidative stress-induced death through bioenergetic improvement
I. Lejri, A. Grimm, M. Miesch, P. Geoffroy, A. Eckert, A.G. Mensah-Nyagan
Biochim. Biophys. Acta 2017, 1863, 631-642 – DOI: 10.1016/j.bbadis.2016.12.007
2016
A grapevine cytochrome P450 generates the precursor of wine lactone, a key odorant in wine
Ilc, T., Halter, D., Miesch, L., Lauvoisard, F., Kriegshauser, L., Ilg, A., Baltenweck, R., Hugueney, P., Werck-Reichhart, D., Duchêne, E. and Navrot, N.
New Phytol. 2016 – DOI: 10.1111/nph.14139
Novel analogs of allopregnanolone show improved efficiency and specificity in neuroprotection and stimulation of proliferation
M. Karout, M. Miesch, P. Geoffroy, St. Kraft , H-D. Hofmann, A.G. Mensah-Nyagan, M. Kirsch
J. Neurochem. 2016 – DOI: 10.1111/jnc.13693
Diastereo- and Enantioselective Synthesis of Polyfunctionalized Diquinanes, Hydrindanes, and Decalins Bearing a Hydroxyl Group at the Ring Junction (invited review by Prof. P. Knochel)
C. F. Heinrich, C. Peter, L. Miesch, P. Geoffroy, M. Miesch
Synthesis 2016, 48, 1607-1615 – DOI: 10.1055/s-0035-1561858
Concise approach to (ent)-14β-hydroxysteroids through highly diastereo/enantioselective Diels-Alder reactions
C. Peter, B. Ressault, P. Geoffroy, M. Miesch
Chem. Eur. J. 2016, 22, 10808-10812 – DOI: 10.1002/chem.201601926
Dynamics of Jasmonate Metabolism upon Flowering and across Leaf Stress Respons Arabidopsis thaliana
E. Widemann, E. Smirnova, Y. Aubert, L. Miesch, T. Heitz
Plants 2016 – DOI:10.3390/plants5010004
Silver-Catalyzed 7-exo-dig Cyclization of Silylenolether-ynesulfonamides
Heinrich, C. F.; Fabre, I; Miesch, L.
Angew. Chem. Int. Ed. 2016, 55, 5170-5174 – DOI: 10.1002/anie.201510708
2015
CYP76C1 (Cytochrome P450)-Mediated Linalool Metabolism and the Formation of Volatile and Soluble Linalool Oxides in Arabidopsis Flowers: A Strategy for Defense against Floral Antagonists
Boachon B., Junker R.R., Miesch L., Bassard J.E., Höfer R., Caillieaudeaux R., Seidel D.E., Lesot A., Heinrich C., Ginglinger J.F., Allouche L., Vincent B., Wahyuni D.S., Paetz C., Beran F., Miesch M., Schneider B., Leiss K., Werck-Reichhart D.
The Plant Cell 2015, 27, 2972-2990 – DOI: 10.1105/tpc.15.00399
Sequential oxidation of jasmonoyl-phenylalanine and jasmonoyl-isoleucine by multiple cytochrome P450 of the CYP94 family through newly identified aldehyde intermediates
E. Widemann, B. Grausem, H. Renault, E. Pineau, C. Heinrich, R. Lugan, P. Ullmann, L. Miesch, Y. Aubert, M. Miesch, T. Heitz, F. Pinot
Phytochemistry 2015, 117, 388-399 – DOI: 10.1016/j.phytochem.2015.06.027
Base catalyzed synthesis of bicyclo[3.2.1]octane scaffolds
Régis Boehringer, Philippe Geoffroy, Michel Miesch
Org. Biomol. Chem. 2015, 13, 6940-6943 – DOI: 0.1039/C5OB00933B
CYP94-mediated jasmonoyl-isoleucine hormone oxidation shapes jasmonate profiles and attenuates defence responses to Botrytis cinerea infection
Yann Aubert, Emilie Widemann, Laurence Miesch, Franck Pinot and Thierry Heitz
J. Exp. Bot. 2015, 66, 3879-3892 – DOI: 10.1093/jxb/erv190
In situ intramolecular catalytic 1,2-addition of allenoates to cyclic ketones towards polycyclic allenoates
Clément F. Heinrich, Michel Miesch and Laurence Miesch
Org. Biomol. Chem. 2015, 13, 2153-2156 – DOI: 10.1039/C4OB02451F
A Route for the Total Synthesis of Enantiomerically Enriched Jasmonates 12-COOH-JA and 12-COOH-JA-Ile
Clément F. Heinrich, Emilie Widemann, Jérémie Sanz, Raphaël Lugan, Thierry Heitz, Franck Pinot, Michel Miesch, and Laurence Miesch
Eur. J. Org. Chem. 2015, 5, 1130-1136 – DOI: 10.1002/ejoc.201403347
Identification of the 12-oxojasmonoyl-isoleucine, a new intermediate of jasmonate metabolism in Arabidopsis, by combining chemical derivatization and LC-MS/MS analysis
E. Widemann, T. Heitz, L. Miesch, M. Miesch, C. Heinrich, F. Pinot, R. Lugan
Metabolomics 2015 – DOI: 10.1007/s11306-014-0754-7
2014
Analysis of sitosteryl oleate esters in phytosterols esters enriched foods by HPLC-ESI-MS2
Julien-David, D.; Zhao, M.; Geoffroy, P.. Miesch, M.; Marchioni, E.
Steroids 2014, 84, 84-91 – DOI: 10.1016/j.steroids.2014.03.013
Dual function of the CYP76 family from Arabidopsis thaliana in monoterpenols and phenylurea herbicides metabolism
Höfer R., Boachon B., Renault H., Gavira C., Iglesias J., Miesch L., Ginglinger J.F., Allouche L., Miesch M., Grec S., Larbat R., Werck-Reichhart D.
Plant Physiol. 2014, 166, 1149 – DOI: 10.1104/pp.114.244814
2013
Arabidopsis ERG28 Tethers the Sterol C4-Demethylation Complex to Prevent Accumulation of a Biosynthetic Intermediate That Interferes with Polar Auxin Transport
Mialoundama, A. S.; Jadid, N.; Brunel, J.; Di Pascoli, T.; Heintz, D.; Erhardt, M.; Mutterer, J.; Bergdoll, M.; Ayoub, D.; Van Dorsselaer, A.; Rahier, A.; Nkeng, P.; Geoffroy, P.; Miesch, M.; Camara, B.; Bouvier, F.
Plant Cell 2013, 25, 4879-4893 – DOI: 10.1105/tpc.113.115576
Gene co-expression analysis reveals a complex metabolism of the monoterpene alcohol linalool in Arabidopsis thaliana flowers
Ginglinger, J-F.; Boachon, B.; Höfer, R.; Paetz, C.; Köllner, T. G.; Lugan, R.; Mutterer, J.;. Fischer, M.; Ullmann, P.; Beran, F.; Claudel, P.; Baltenweck, R.; Miesch, L.;. Verstappen, F.; Bouwmeester, H.; Miesch, M.; Schneider, B.; Gershenzon, J.; Ehlting, J.; Werck-Reichhart D.
Plant Cell 2013, 25, 4640-4657 – DOI: 10.1105/tpc.113.117382
Phosphine-Catalyzed Reactions of Activated Olefins Tethered to Cycloalkanones. Substrate- and Solvent-Controlled Synthesis of Bicyclo[3.2.1]octanones, Mixed Acetals, and Morita-Baylis-Hillman Products
Wang, Y.; Jaunet, A.; Geoffroy, P.; Miesch, M.
Org. Lett. 2013, 15, 6198-6201- DOI: 10.1021/ol403039b
Total syntheses of Hamigeran B
Miesch, M.; Welsch, T.; Rietsch, V. Miesch, L.
Strategies and Tactics in Organic Synthesis 2013, 9, 203-229 – DOI: 10.1016/B978-0-08-099362-1.00007-2
The amido-hydrolases IAR3 and ILL6 contribute to jasmonoyl-isoleucine hormone turnover and generate 12-hydroxy-jasmonic acid upon wounding in Arabidopsis leaves
E. Widemann, L. Miesch, R. Lugan, E. Holder, C. Heinrich, Y. Aubert, M. Miesch, F. Pinot, T. Heitz
J. Biol. Chem. 2013, 288, 31701-31714 – DOI: http://www.jbc.org/content/288/44/31701
14β-Hydroxypregnanes from Succulent Plants Belonging to Hoodia gordonii and Caralluma Genus: Extraction, Biological Activities, and Synthesis
Yatin J. Shukla, Ikhlas A. Khan, Philippe Geoffroy, Michel Miesch
Studies in Natural Products Chemistry 2013, 40, 327-358 – DOI: 10.1016/B978-0-444-59603-1.00009-6
Stereoselective cross aldol condensation of bicyclo[3.2.0]alkanones
Miesch, L.; Welsch, T.; Miesch, M.
Org. Biomol. Chem. 2013, 11, 4025-4029 – DOI: 10.1039/C3OB40431E
2012
Derivatives of allopregnanolone and of epiallopregnanolone, their preparation and their uses for treating a neuropathological condition
Mensah-Nyagan, Ayikoe Guy; Meyer, Laurence; Patte-Mensah, Christine; Taleb, Omar; Miesch, M; Geoffroy, P; Ressault, Bl.
PCT Int. Appl. (2012), WO 2012127176 A1 20120927
New sterol derivatives as inhibitors of ABC and PIN transporters and their preparation and use
Camara, B.; Bouvier, F.; Heintz, D.; Miesch, M.; Geoffroy, P.
French Patent Application. Demande (2012), FR, FR 2976578 A1 20121221
A Silver Catalyzed Spirocyclization of Alkynyl Silyl Enol Ethers
Schäfer, C.; Miesch, M.; Miesch, L.
Chem. Eur. J. 2012, 18, 8028-8031 – DOI: 10.1002/chem.201200116
Fate of polyphenols and antioxidant activity of barley throughout
Leitao C., Marchioni E., Bergaentzlé M., Zhao. M., Didierjean L., Miesch L., Holder E., Miesch M., Saïd Ennahar S. J.
Cereal Science 2012, 55, 318-322 – DOI: 10.1016/j.jcs.2012.01.002
Method for the synthesis of steroids
Miesch, M.; Ressault, B.; Geoffroy, P.
French Patent Application. Demande (2012), FR 2963347 A1 20120203; PCT Int. Appl. (2012), WO 2012022880 A2 20120223
Intramolecular reductive ketone-alkynoate coupling reaction promoted by (η2-propene)titanium
Schäfer, C.; Miesch, M.; Miesch, L.
Org. Biomol. Chem. 2012, 10, 3253-3257 – DOI: 10.1039/C2OB07049A
Cytochromes P450 CYP94C1 and CYP94B3 catalyze two successive oxidation steps of the plant hormone jasmonoyl-isoleucine for catabolic turnover
Heitz, T.; Widemann, E.; Lugan, R.; Miesch, L.; Ullmann, P.; Désaubry, L.; Holder, E.; Grausem, B.; Kandel, S.; Miesch, M.; Werck-Reichhart D.; Pinot, F.
J. Biol. Chem. 2012, 287, 6296-6306 – DOI: 10.1074/jbc.M111.316364
Access to Polyfunctionalized Diquinanes, Hydrindanes, and Decalines via TiCl4 Promoted Michael-Aldol and Baylis-Hillman Reactions
Ressault, B.; Jaunet, A.; Geoffroy, P.; Goudedranche, S.; Miesch, M.
Org. Lett. 2012, 14, 366-369 – DOI: 10.1021/ol203118t
